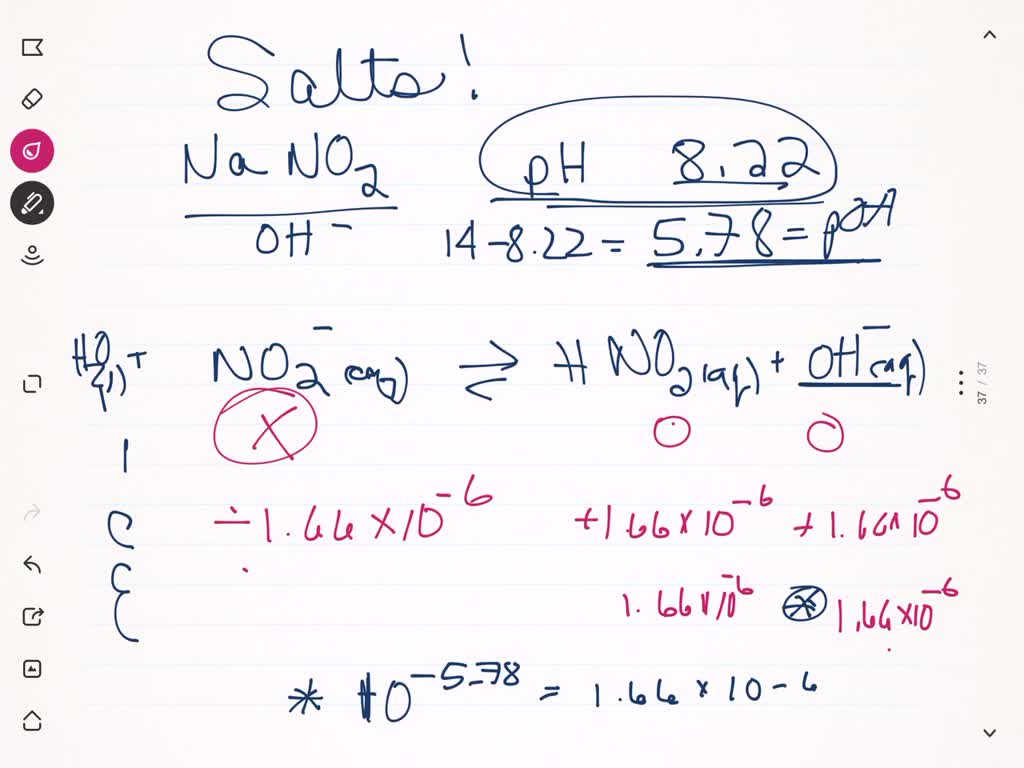The influence of pH on the peak potential and the peak current of NaNO2... | Download Scientific Diagram

50 mL of 2N acetic acid mixed with 10 mL of 1N sodium acetate solution will have an approximate pH of: (Ka = 10^-5) .

Atmosphere | Free Full-Text | Aqueous-Phase Brown Carbon Formation from Aromatic Precursors under Sunlight Conditions
Guy in my chemistry group did this as an answer to the question: Determine the pH of a solution formed by adding 1.0 g of NaNO2 to 750 mL of water. We

SOLVED: The pH of a solution of NaNo2 and HNO2 is 8.05. What is the molarity of HNO2 if the molarity of NaNO2 is 0.011M?

The ionization constant of nitrous acid is 4.5 × 10 4 . Calculate the pH of 0.04 M sodium nitrite solution and also its degree of hydrolysis.

Larutan NaNO2 0,02 M 200ml mempunyai pH sebesar .... (Ka HNO2 = 4,5 . 10^-4) Mohon penjelasannya - Brainly.co.id

The final major product of the reaction is : Ph - underset(underset(Ph)(|)) overset(overset(OH)(|))C - underset(underset(NH2)(|))CH - CH3 overset(NaNO2 + "HCI") (to)
What is the pH of a 0.40 M solution of sodium nitrite, NaNO2? The pKa for nitrous acid (HNO2) is 3.35 - Quora

SOLVED: What is the pH of a buffer containing 0.225 M NaNO2 and 1M HNO2 . The Ka for the acid is 5.6 x 10-4.