SOLVED: Problem Solving: What are the pH values of (a) 2.0 × 10−2 mol/L KOH and of (b) 2.0 × 10−6 mol/L KOH?

The pH of Aqueous NaOH/KOH Solutions: A Critical and Non-trivial Parameter for Electrocatalysis | ACS Energy Letters

OneClass: What are the pH and pOH for a 0.025 M KOH solution? A) pH = +1.60 and pOH =+12.40 B) pH = +...
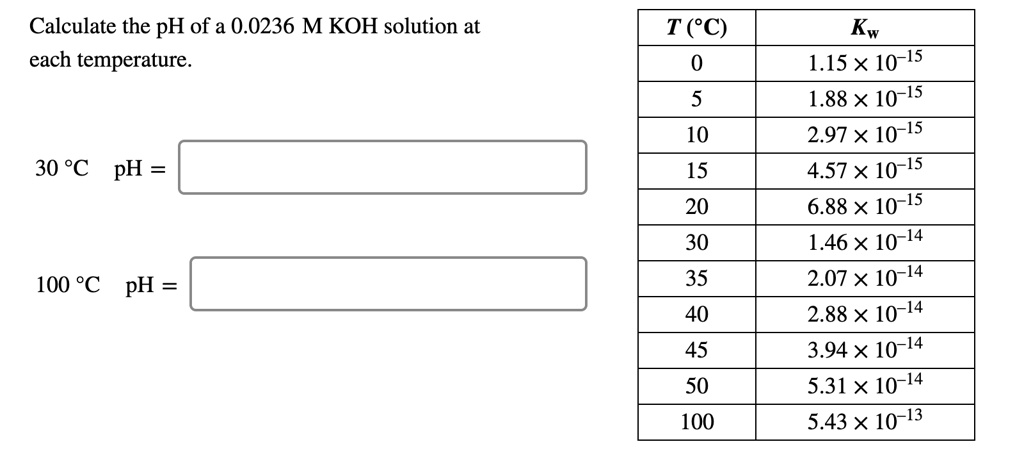
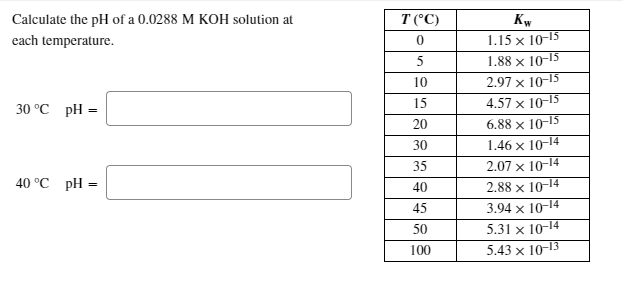
SOLVED: Calculate the pH of a 0.0236 M KOH solution at each temperature. T(C) Kw 1.15 X 10-15 1.88 X 10-15 2.97 X 10-15 4.57X 10-15 6.88 X 10-15 1.46 X 10-14 2.07 X 10-14 2.88 X 10-14 3.94 X 10-14 5.31X 10-14 5.43X 10-13 10 15 20 30 35 40 45 50 100 30 ...
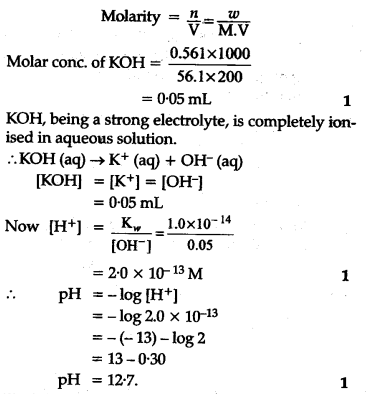
If 0-561 g of KOH is dissolved in water to give 200 mL of solution at 298 K. Calculate the concentrations of potassium, hydrogen and hydroxyl ions. What is its pH? -

TRACCIA 9 Calcolare il pH di una soluzione ottenuta sciogliendo 12 g di NaH2PO4 in 100 ml di KOH 2 M. (H3PO4: Ka1=7,6x10-3, Ka2=6,2x10-8, Ka1=4,4x10-13). - ppt scaricare

SOLVED:A solution of KOH has a pH of 13.29 . It requires 27.66 mL of 0.2500 MHCl to reach the equivalence point. (a) What is the volume of the KOH solution? (b)
Calculate the pH of a solution obtained by mixing 2 ml of HCl of pH 2 and 3 ml of solution of KOH of pH = 12 - Sarthaks eConnect | Largest Online Education Community

50 ml 01m koh is mixed with 50 ml 01m hcooh ph of the final solution is 6bh2onss -Chemistry - TopperLearning.com