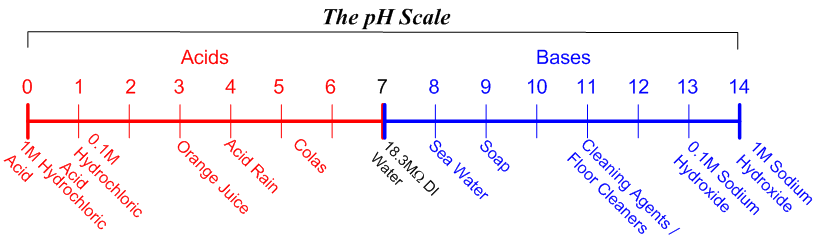
Calculate the pH of a solution which contains 9 9ml of 1M HCL and 100ml of 0 1M NaOH - Chemistry - Equilibrium - 12574567 | Meritnation.com
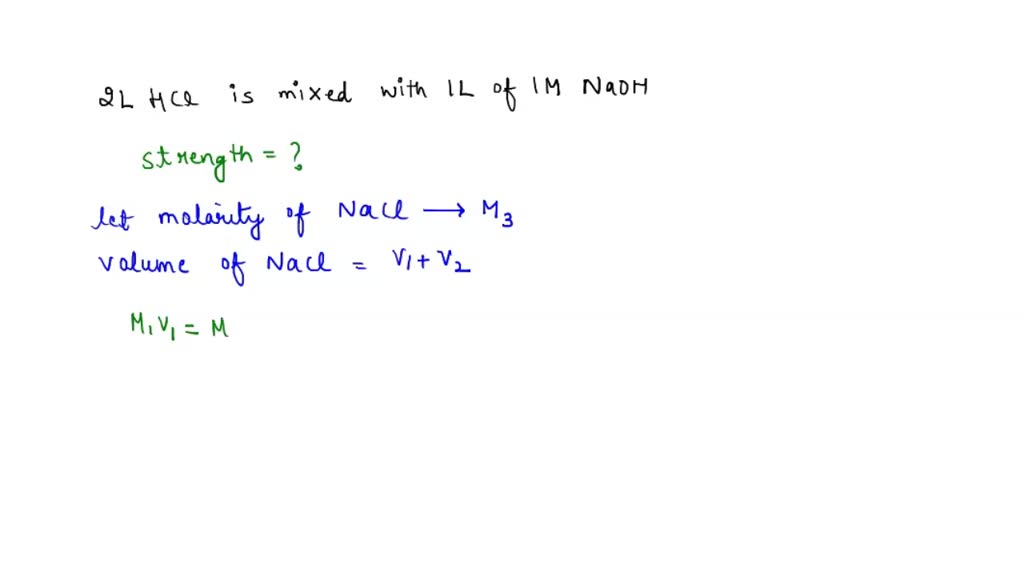
SOLVED: two litres of 1M Hcl is mixed with 1 litre of 1M NaOH Solution.Calculate strength of salt formed and PH of resulting solution. Ans : Strength = 0.33M ,PH=0.48

Calculate the `pH` of a solution which contains `10 ml` of `1 M HCl` and `10 ml` of `2M NaOH` - YouTube
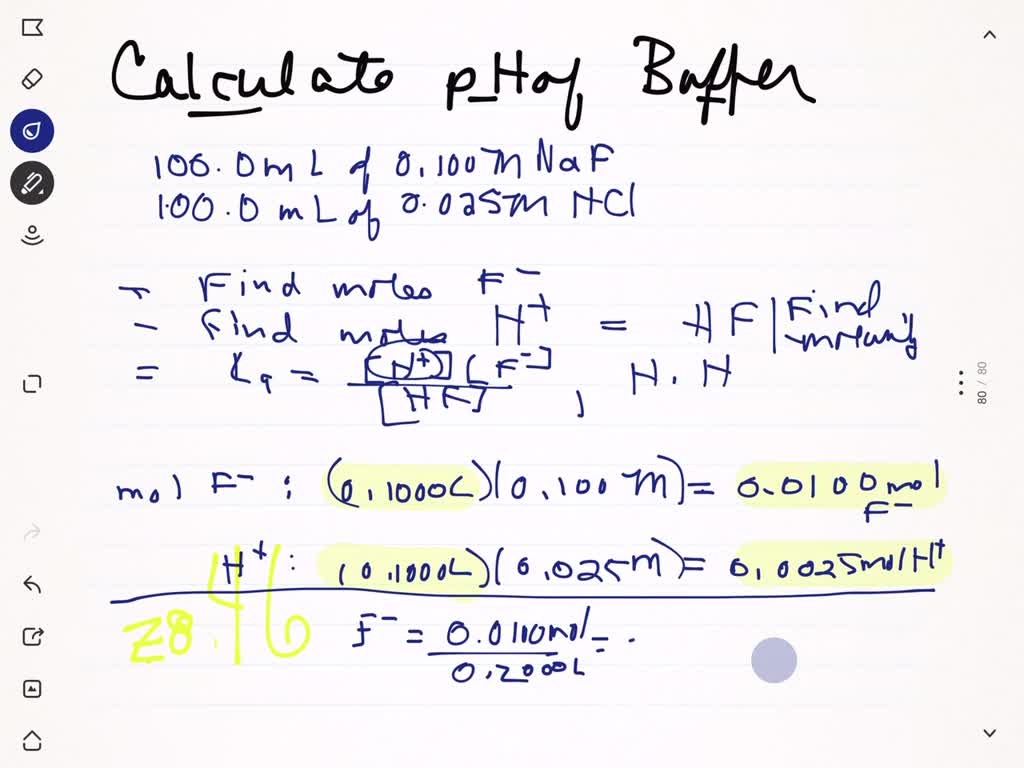
SOLVED: 6. Calculate the expected pH of the DI H2O + 1.0 mL of 1M HCl *(The final volume after the addition of HCl is 51 mL)* 7. Calculate the expected pH

The changes in pH after additon of various volumes of 1M HCl and 1M... | Download Scientific Diagram






![BT022] 1M Tris-HCl, pH 9.0 | Biosolution BT022] 1M Tris-HCl, pH 9.0 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2015/05/BT016-1M-Tris-HCl.jpg)