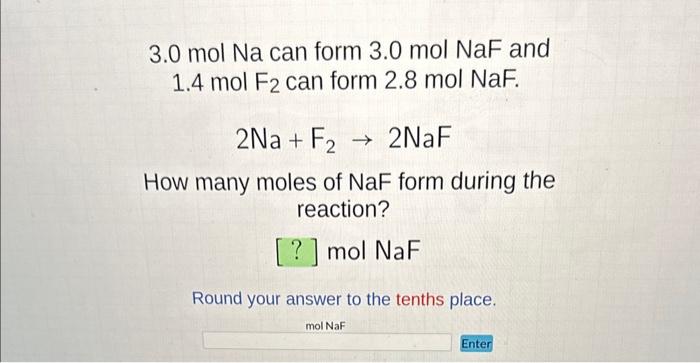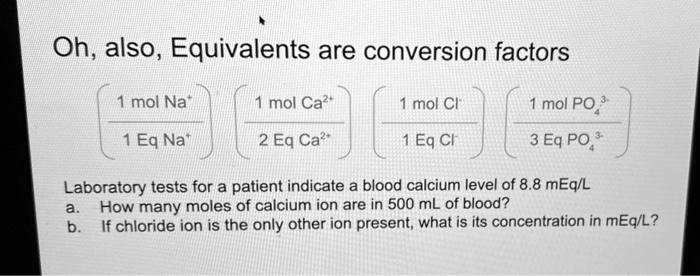
SOLVED: Oh, also, Equivalents are conversion factors mol Na mol Ca? mol Cl mol PO Eq Na- 2 Eq Ca? 1 Eq Cl 3 Eq PO; Laboratory tests for a patient indicate

The energy change for the alternating reaction that yields chlorine sodium (Cl^+Na^-) will be: 2Na(s) + Cl2(g) → 2Cl^+Na^-(s) Given that:Lattice energy of NaCl = - 787 kJ mol^-1 Electron affinity of

Come possiamo conoscere il numero di atomi o molecole presenti in una definita quantità di sostanza? - ppt scaricare

Esercizio quantità chimica (moli), numero particelle e Avogadro, N = n ∙ NA, 1 di 2, lezione chimica - YouTube

Voltammograms CV and DPV of 1.0 Â 10 À4 mol L À1 paraquat in 0.1 mol L... | Download Scientific Diagram

Esercitazione 1 - MASSA ATOMICA, MASSA MOLECOLARE, MOLE. COMPOSIZIONE PERCENTUALE, FORMULA MINIMA. m - Studocu

Come possiamo conoscere il numero di atomi o molecole presenti in una definita quantità di sostanza? - ppt scaricare
Avogadro number NA is changed fromx 1023 mol 1 to 6.022 x 1020 mol 1, this5 IfRe AIPMT 2015would changeThe ratio of chemical species to each other ina balanced equation13(2) The ratio

Avogadro's Number Avogadro's number (symbol N) is the number of atoms in grams of carbon. Its numerical value is 6.02 × Therefore, a - ppt download

Acetic acid, sodium salt, 99+%, for mol biology, anhydrous, DNAse, RNAse and P, Thermo Scientific Chemicals | Fisher Scientific