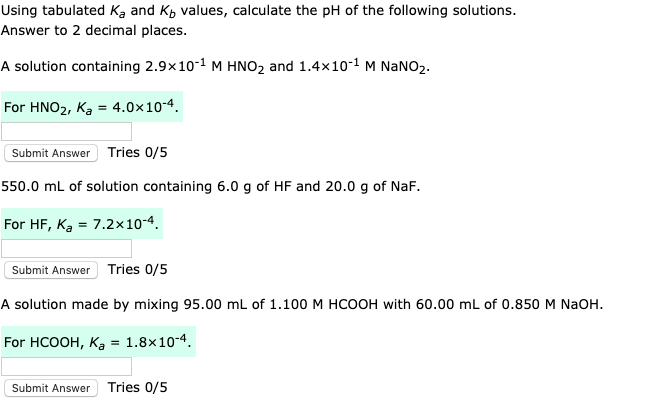
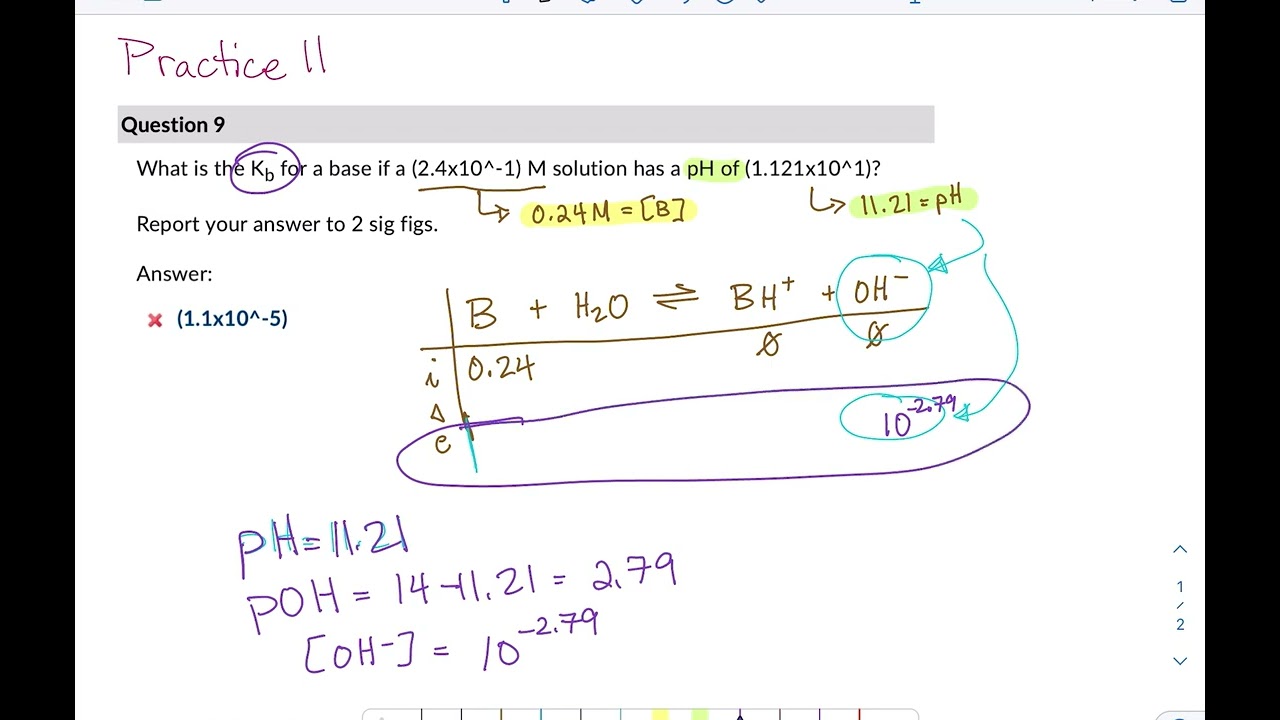
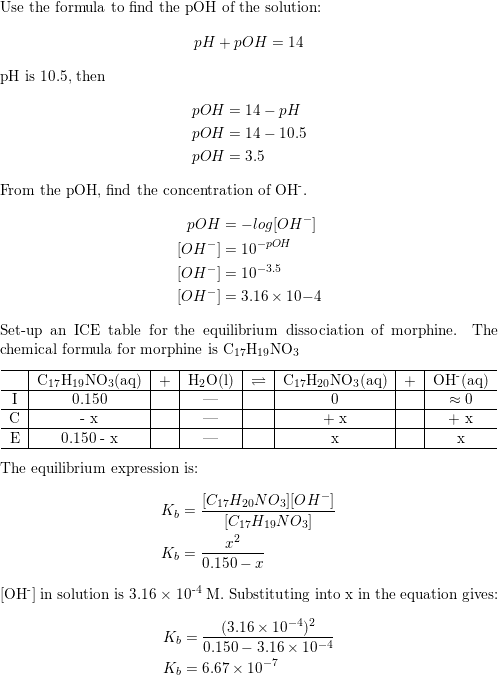
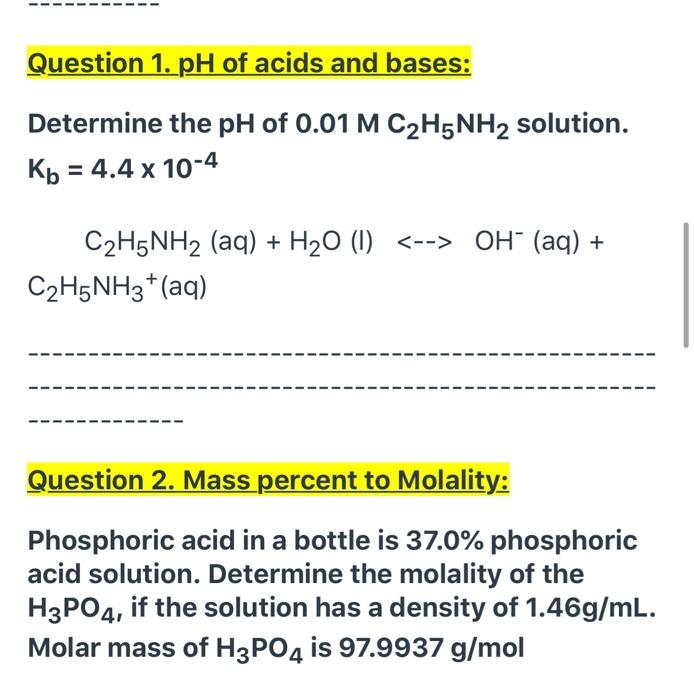
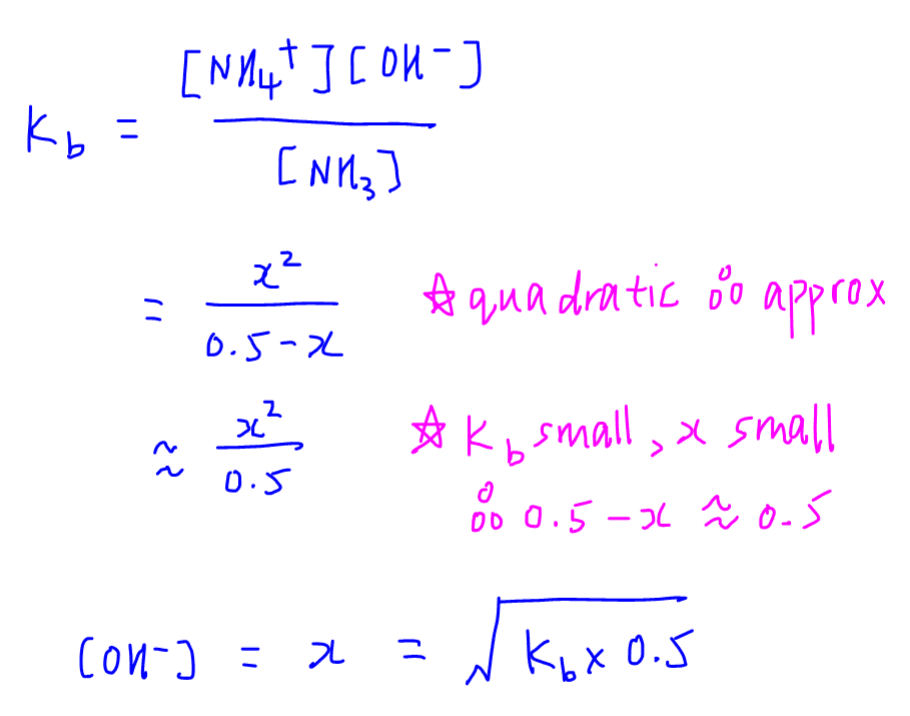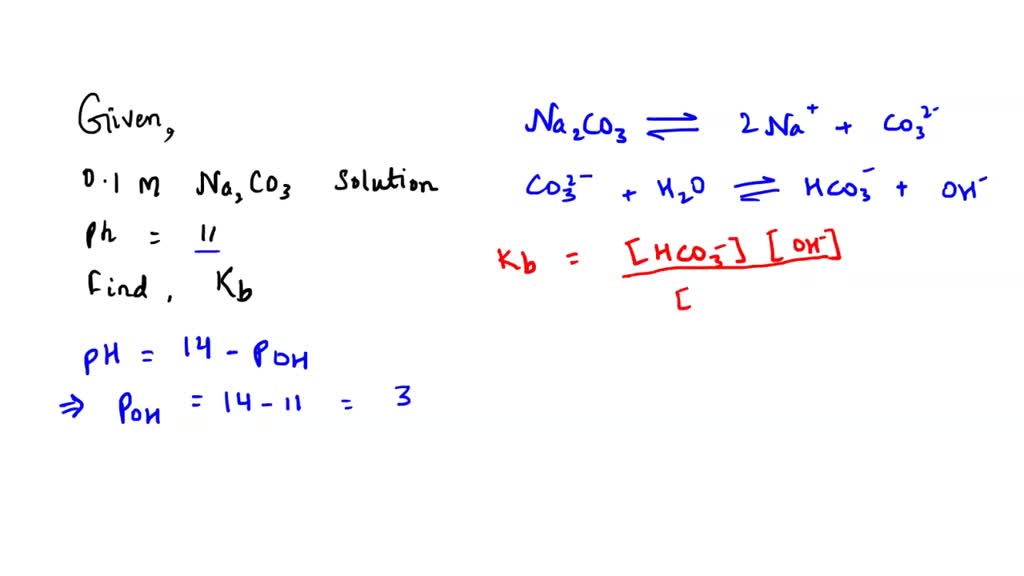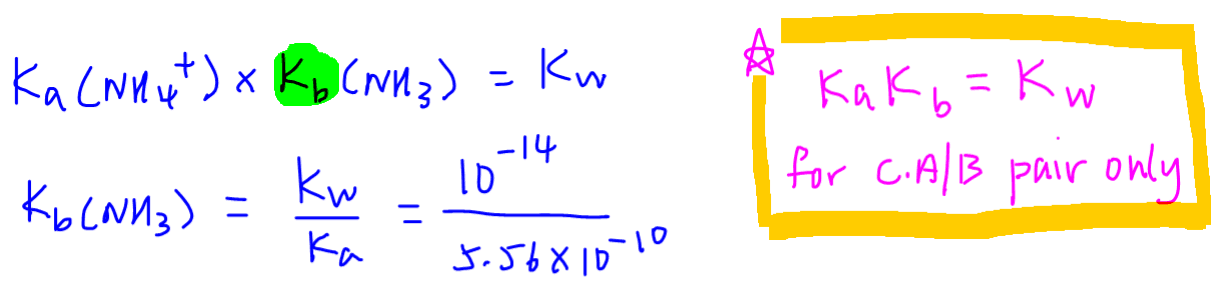Calculate the pH of a 0.10 M solution of hydrazine, N2H4. Kb for hydrazine is 1.3×10−6 - Home Work Help - Learn CBSE Forum

The pH profiles for the V 1 /K B function with neutral substrates. (A)... | Download Scientific Diagram

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - Yo… | Chemistry lessons, Chemistry classroom, Chemistry basics
Calculate pH of the following mixtures. Given that Ka = 1.8 × 10^–5 and Kb = 1.8 × 10^–5: - Sarthaks eConnect | Largest Online Education Community
![SOLVED: Helpful Equations pH = -log [HzO+ ] Kw = Ka X Kb [ATJ[Hao+1 [HA] = pOH -log [OH:] 14 = pH + pOH [HA][OH-] [A-] Kb Henderson- Hasselbalch Equation [A-] pH =pKa log - [HA] SOLVED: Helpful Equations pH = -log [HzO+ ] Kw = Ka X Kb [ATJ[Hao+1 [HA] = pOH -log [OH:] 14 = pH + pOH [HA][OH-] [A-] Kb Henderson- Hasselbalch Equation [A-] pH =pKa log - [HA]](https://cdn.numerade.com/ask_images/9f4de4f19628424f8aea53cff3ecadcb.jpg)
SOLVED: Helpful Equations pH = -log [HzO+ ] Kw = Ka X Kb [ATJ[Hao+1 [HA] = pOH -log [OH:] 14 = pH + pOH [HA][OH-] [A-] Kb Henderson- Hasselbalch Equation [A-] pH =pKa log - [HA]
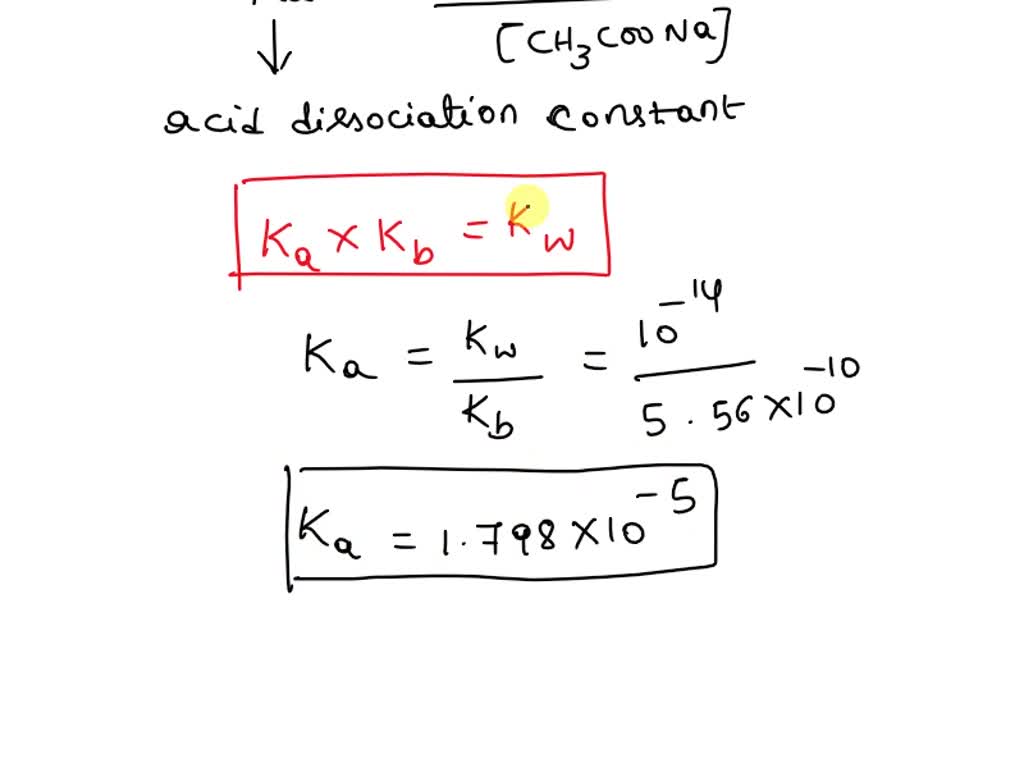
SOLVED: Calculate the pH of a 0.40 M CH3COONa solution. Kb for CH3COO- = 5.6x10-10 (Show the dossociation equation for the salt. Show the reaction of CH3COO- ion with H2O.)

![Calculating [OH-], pH and pOH from Kb Calculating [OH-], pH and pOH from Kb](https://www.mi.mun.ca/users/pfisher/chemistry1011_135/img007.gif)