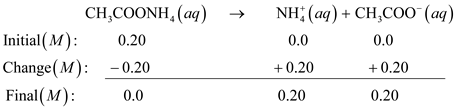
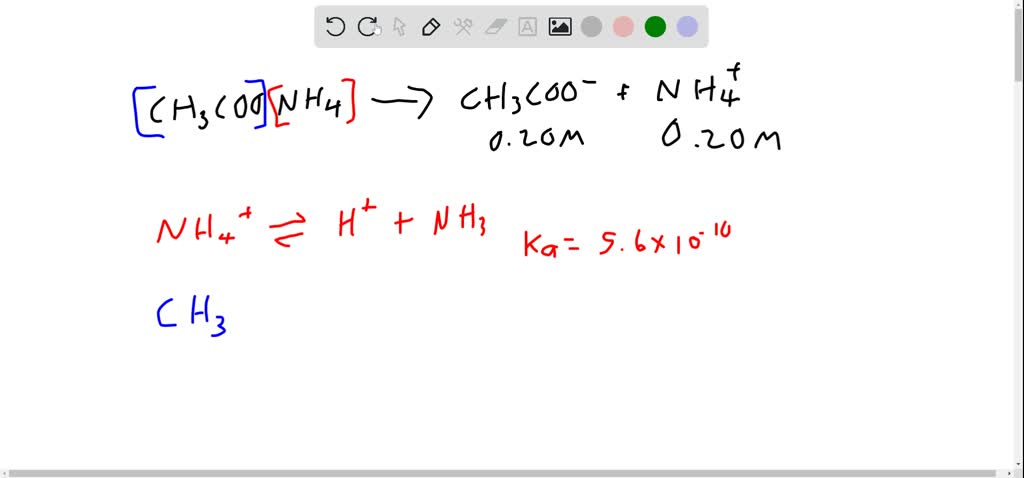
Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.
Identity correct statement (a)degree of hydrolysis decrease on doubling the concentration of aqueous solution of CH3COONH4 (b)for 1M CH3COOH pH=pKa/2 (c)salt hydrolysis depends on size of atom (d)all

Which of the following solutions will be acidic?(1) 0.1M FeSO4 (2) 0.1M (NH4)2SO4 (3) 0.1M CH3COONa (4) 0.1M NH4OH

Chromatograms of drugs obtained using the suitable columns packed with... | Download Scientific Diagram
![Calculate the extent of hydrolysis and the pH of 0.02 M CH3COONH4. [ Kb (NH3) = 1.8 × 10^- 5,Ka (COOH) = 1.8 × 10^- 5 ] Calculate the extent of hydrolysis and the pH of 0.02 M CH3COONH4. [ Kb (NH3) = 1.8 × 10^- 5,Ka (COOH) = 1.8 × 10^- 5 ]](https://d1hj4to4g9ba46.cloudfront.net/questions/1200266_999624_ans_e02120991ce8408c94c67fa9a8ab69a7.jpg)
Calculate the extent of hydrolysis and the pH of 0.02 M CH3COONH4. [ Kb (NH3) = 1.8 × 10^- 5,Ka (COOH) = 1.8 × 10^- 5 ]

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.
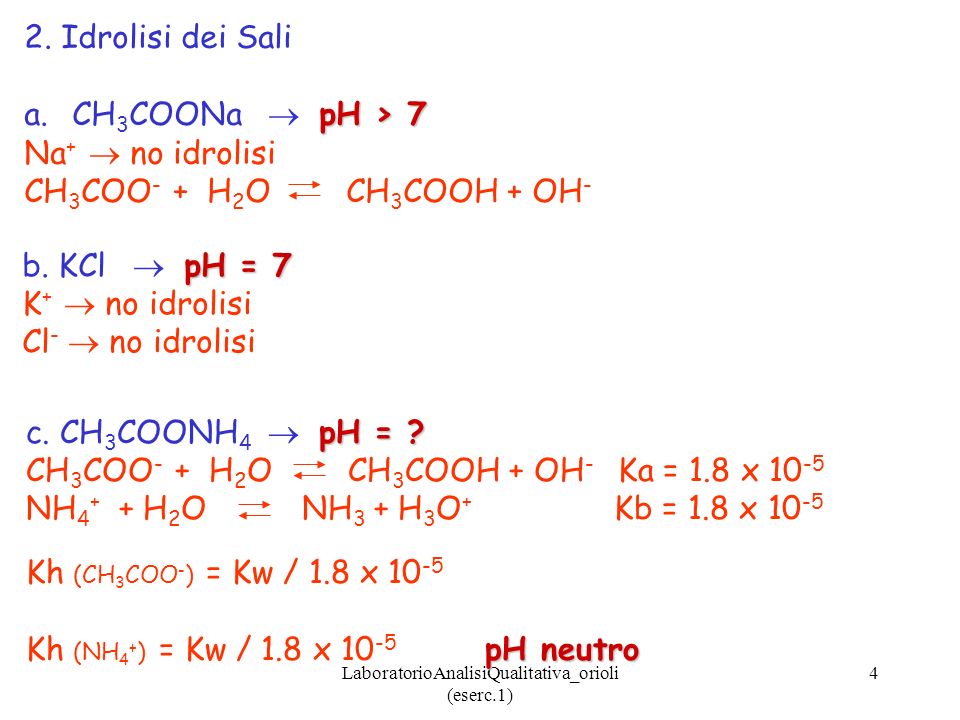
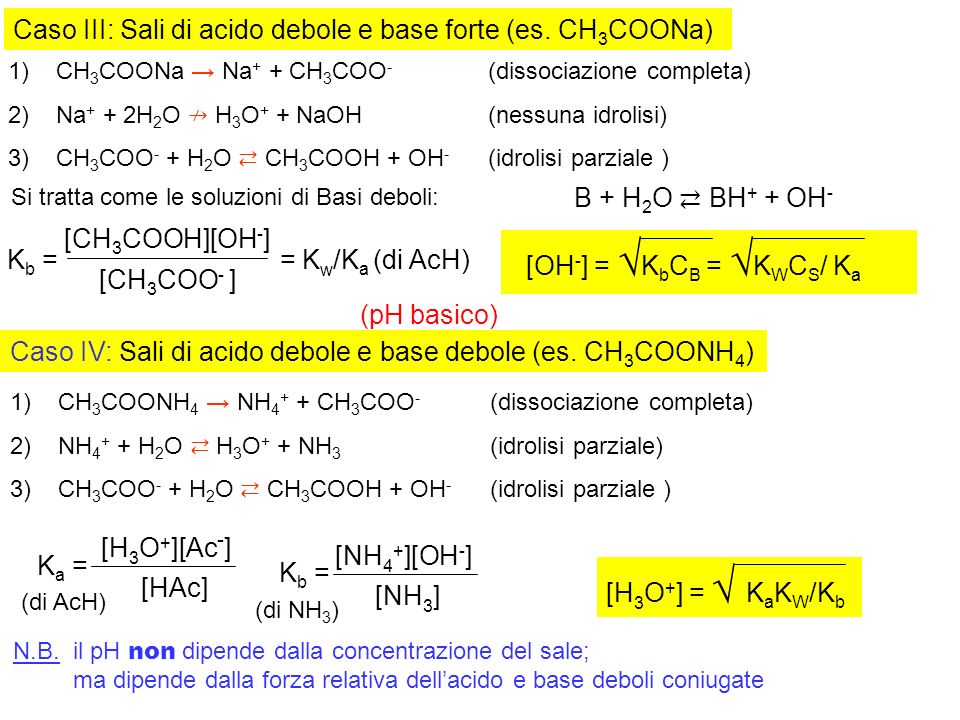
110 Cap.11 Reazioni di idrolisi - Soluzioni saline ESEMPIO 1. Soluzione di acetato di sodio. Ac– + H2O ←→ AcH + OH–

Calculate the percent hydrolysis of 0.02M of CH3COONH4 .Kb(NH3) = 1.6 × 10^-5 & Ka(CH3COOH) = 1.6 × 10^-5

WHICH OF THE FOLLOWING INCREASING ORDER OF PH OF .1 M SOLUTION OF THE COMPOUND A-HCOONH4,B-CH3COONH4 - Brainly.in

Hitunglah pH larutan CH3COONH4 0,5 M, jika diketahui Ka CH3COOH =10 pangkat min 11, Kb NH3 = 10 pangkat - Brainly.co.id

Ammonium acetate, Puriss. p.a., ACS Reagent, Reag. Ph. Eur., 98 , Honeywell Fluka | Fisher Scientific
![pH of a solution of 0.1 M [CH3COONH4(aq)] is [given: Ka(CH3COOH) = Kb(NH4OH) = 1.8 x 10^-5)] pH of a solution of 0.1 M [CH3COONH4(aq)] is [given: Ka(CH3COOH) = Kb(NH4OH) = 1.8 x 10^-5)]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/644382825_web.png)